
DOES IT WHISTLE, RING, ROAR OR DRONE IN YOUR EARS?

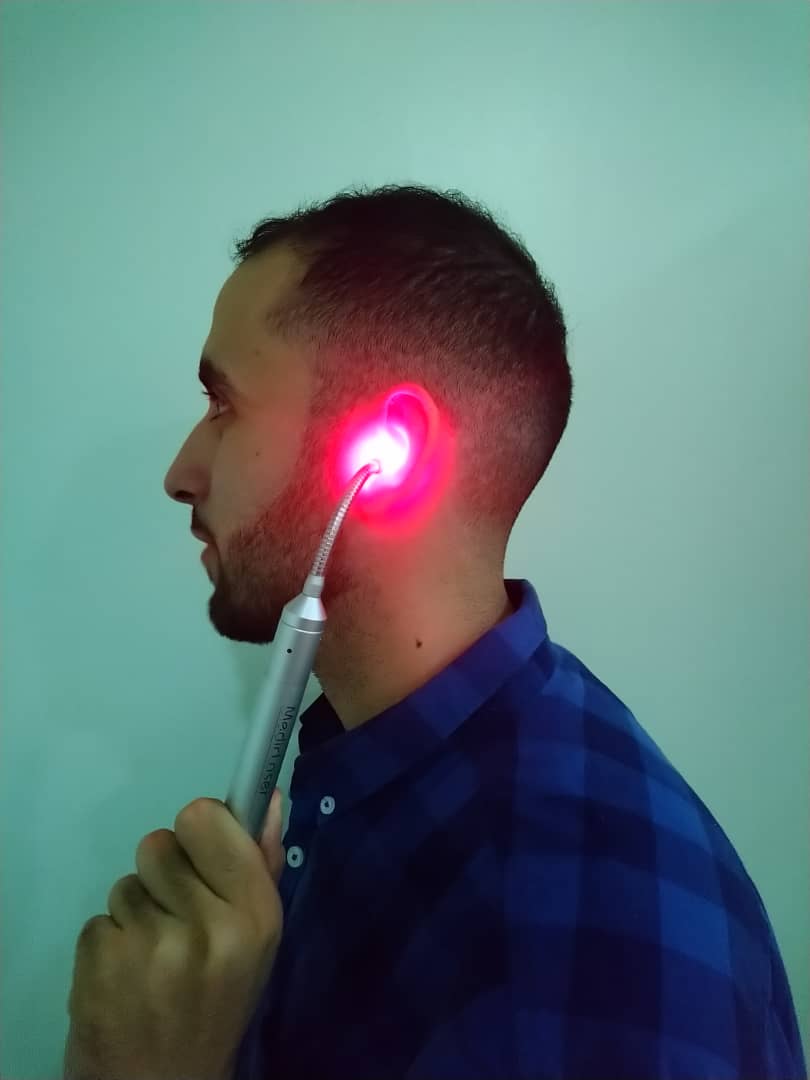
The treatment with the EarLaser4 is completely safe and painless. The laser beam even penetrates the deeper subcutaneous layers and works as curative bio-stimulation directly on the metabolism in the connective tissue. This leads to rapid regeneration of the hearing cells, stimulation of the immune system, acceleration of cell division and activation of specific defensive molecules. More than 40’000 affected persons have already been successfully treated with this system.
Many disorders arise through a deficiency of ATP (adenosine triphosphate), the cell energy necessary for life. ATP is a key substance in our cells. The mitochondria (cell power-plants) provide ATP energy for the cell processes through glucose combustion. ATP deficiency leads to cell damage and destruction in the end, but concentrated EarLaser4 light (660 nm wavelength) stimulates glucose combustion to improve the ATP supply. The EarLaser4 therapy can therefore accelerate the regeneration of damaged cells in the auditory system.
The principle of EarLaser4 is to project the needed energy concentratedly and precisely on the ailing area by means of an elaborate fiberglass cable. With the optimized EarLaser4; we set new standards regarding product quality and ease of handling. Due to the perfect positioning of the EarTool the laser beam can reach the inner ear more exactly.
The laser treatment underwent successful long-term testing in hospitals and in physician practices. It was submitted to extensive medical tests and proven scientifically by various medical studies. The safety and effect of the EarLaser4 were attested and certified medically.
Suitable with symptoms like:
– Acute and chronic tinnitus since 3 years
– Partial deafness due to tinnitus
– Morbus Ménière (dizziness)
– Acute hearing loss and hearing distortion
– Consequences of a middle ear inflammation
– Circulatory problem in the inner ear
TECHNICAL DATA
Output power: 100 mW
Wavelength: 660 nm
Treatment time: ca. 2 minutes per day
Skin penetration: ca. 3 cm
Warranty: 2 years
Battery operation: 2 x 3.5V (CR123)
Laser protection class: 1
Medical device class: IIa
Medical device testing: CE (Europe), CB (International), ETL (USA/Canada)
Patents: CH (699352), EU (06761280.4), Intern. (PCT/CH 2006/000429)
OPERATION INSTRUCTIONS
The MedicLaser in conjunction with the EarTool can be used for the following applications:
| • Ear pressure: | 1 x per day, 2 minutes, for at least 3 weeks |
| • Tinnitus: | 1 x per day, 2 minutes, for at least 10 weeks |
There is an extensive instruction manual enclosed, which explains all treatment methods simply and in detail.
♦ The treatment time may be extended, depending on the degree of severity.
CERTIFICATES AND ACCREDITATION
Our products and our business are subject to the most scrupulous ISO 13485 quality checks. Both have been certified in accordance with the strict guidelines for medical devices. We have CE marking and operate in accordance with EU guidelines 93/42/EWG. Our work is supervised by the Swiss accreditation body and is therefore directly affiliated to the European Accreditation System.
TinniTool ProfiLaser meets requirements as per:
– ISO 13485 + 14971
– CE (Europa) 93/42/CEE
– CB (Internazionale) IEC-EN 60601
– ETL (USA/ Canada) UL 60601
– IEC 60601-1 = Medical electrical equipment: General requirements for safety
– IEC 60601-1-2 = Medical electrical equipment: General specifications for safety
– IEC 60601-1-6 = Medical electrical equipment: General requirements for basic safety and essential performance – Col-lateral standard: Usability
– IEC 60601-1-11 = Medical electrical equipment: General requirements for basic safety and essential performance
– IEC 60825-1 = Safety of laser devices: classification of facilities and requirements
– IEC 62366 = Medical devices – Application of usability engineering to medical devices
– IEC 980 = Graphical symbols for labeling of medical devices
– Risk analysis in accordance with DIN-EN-1441 and EN-ISO-14971
CLINICAL STUDIES
Evaluation of the customer satisfaction among users of the TinniTool MedicLaser
Finn Andersen, Gabriele Deterville, IHA-GfK, ISO-certified (ISO-9001), in accordance with the standards of Swiss-Interview
Low-level laser therapy for the treatment of tinnitus with TinniTool EarLaser
Dr. Domenico Cuda, Dr. Antonio R. de Caria, ENT Department, Piacenza Clinic, Italy / Published in the “International Tinnitus Journal” Vol.14, No.2, 175-180 (2009)
The effectiveness of soft laser therapy for tinnitus and sensory hearing loss
Dr. Mohammad Al-Masri, Ph.D.; Lina Abu Khader, MSc., Mohammad Tawalbeh, AL-Ahliyya Amman University
TinniTool Low level laser therapy in patients with complaints of tinnitus
Ahmed H Salahaldin, Khalid Abdulhadi, Nihal Najjar, General Hospital, Hamad Medical Corporation, Qatar
TinniTool Low Level Laser Therapy: Tinnitus subjective characteristics and measurements
Prof. M. Savastano (MD), L. Termine (Tech), V. Prosenikliev (MD), University of Padua (IT)
Low-level laser therapy for tinnitus
Peng Z, Yiu-Qi Chen, Head and Neck Surgery, Beijing Tongren Hospital, Capital Medical University, China
Low-level-laser-therapy in patients with chronic cochlear dysfunction
Dr. Stefan Tauber et al., HNO-Universitätsklinik und Laser Forschungslabor, Universität München
Neural correlates of transmeatal cochlear laser (TCL)
Christian M. Siedentopf, Department of Radiology II, Division of Neuroradiology, University Hospital of Innsbruck, Austria
Ex-vivo laser penetration study
Dr. Beyer, Dr. Tauber
Comprehensive therapy of patients suffering from Tinnitus
M. Prochazka, R. Tejnska
REFERRENCES
(You will be redirected into mymuna.com)
